Preclinical Trials
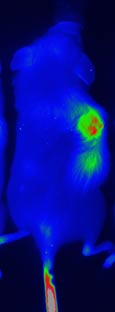
NanoMed has successfully developed a preclinical programme and demonstrated safety and efficacy in animal studies for its Gemcitabine prodrug nanomedicine. Gemcitabine continues to play a central role in chemotherapy treatment for pancreatic cancer. It is also used for the treatment of a range of other cancers, including colorectal and ovarian cancers, non-small cell lung cancer, bladder cancer and metastatic breast cancer, either as a single agent or in combination therapy. Our primary goal is to quickly complete the preclinical pharmacology studies in rodents and address toxicity and efficacy of our Gemcitabine prodrug nanomedicine in a bigger animal model before moving to clinical phase I trials in humans. NanoMed has also completed preclinical toxicity and efficacy studies in mice for two additional prodrug nanomedicines in its pipeline.
CONTACT
Lane Cove West Business Park
Unit 2, 11/13 Orion Road,
Lane Cove West NSW 2066,
Australia
Telephone: +61 2 9418 6050
E-mail: info@nanomed.com.au

